Norman Goldberg and Wilhelm Graf von der Schulenburg
Institut für Organische Chemie der Technische Universität
Braunschweig
Hagenring 30, D-38106 Braunschweig, Germany
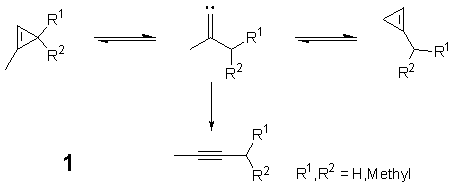
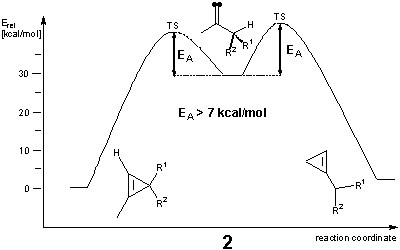
Several experimental studies of cyclopropene rearrangements have lead to strong evidence for the involvement of vinylidenes as reactive intermediates during the thermal ring opening and interconversion of isomeric cyclopropenes (Scheme 1).[1] However, the relative stabilities of the vinylidene intermediates could not be assessed in these experimental studies. We have therefore carried out a theoretical investigation employing high-level density functional - and ab initio -methods to further elucidate the pathways for these cyclopropene isomerizations.
Angew. Chem., Int. Ed. Engl. 1997, 36, 381-383.
[2] W. Graf von der Schulenburg, H. Hopf, R. Walsh, Unpublished results.
[3] N. Goldberg, W. Graf von der Schulenburg, Chem. Commun. 1998, 2761-2762 (incl. Suppl.Mat.).