by Wilhelm Graf von der Schulenburg* and Henning Hopf
Institut für Organische Chemie der Technische Universität
Braunschweig, Germany
and
Robin Walsh
Department of Chemistry, University of Reading, UK.
The isomeric 1,3-dimethylcyclopropene and 1-ethylcyclopropene, and the
isomeric 1,3,3-trimethylcyclopropene and 1-isopropylcyclopropene have been
synthesised and the kinetics of their thermal isomerisations individually
investigated in the gas-phase. Pyrolyses of each cyclopropene generates
the other isomer as a transient species in quantities of several percent
(up to 15%). This cyclopropene-to-
cyclopropene interconversion demonstrates clearly the involvement of
vinylidene intermediates in thermal cyclopropene isomerisations.[1]
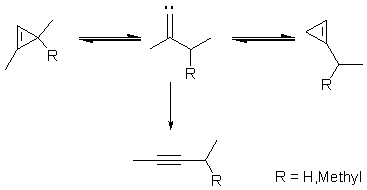
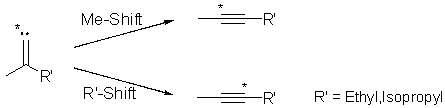
Details of results and their further implications will be discussed at the meeting.
[1] H. Hopf, W. Graf von der Schulenburg, R. Walsh,
Angew.
Chem.
1997,
109, 415-417;
Angew. Chem., Int. Ed. Engl.
1997,
36,
381-383.
[2] W. Graf von der Schulenburg, H. Hopf, R. Walsh,
Unpublished results.
[3] W. Graf von der
Schulenburg, N. Goldberg, Unpublished results.