|
Project area A: Synthesis
Project A1: Violacein as template for chemical diversification
and their biological evaluation
Prof. Stefan Schulz, TU Braunschweig, Institut für Organische Chemie
Web address:
http://www.oc.tu-bs.de/schulz/index_en.html
Violacein is a bacterial pigment produced by bacteria from
tryptophane. Several important biological activities have been
reported, including antibiotic, antiviral, and antitumor activities.
Biotechnological optimization by the cooperating group of Prof.
Wittmann enabled the production of larger amounts. This product will
be the starting material for chemical derivatization to produce
derivatives with enhanced properties. Furthermore, pathway
modification by the Wittmann group may lead to block mutants that
produce derivatives also available for modification. Further
cooperation with other groups will pin down detailed activities in
several biological systems.
Required qualification: Master in Chemistry
Project A2: Chemoenzymatic synthesis of an
antineoplastic polyketide natural product
Dr. Frank Hahn, Leibniz University Hannover, Institute of Organic
Chemistry
Web address:
http://www.oci.uni-hannover.de/de/arbeitskreise/hahn/index.php
This project aims to combine the advantages of enzyme catalysis and
organic synthesis in order to achieve better access to
pharmaceutically relevant natural products. The student will develop
a chemo-enzymatic total synthesis of a polyketide natural product.
He/She will apply and optimize enzymes from polyketide biosynthesis
for a biocatalytic application in crucial synthetic steps. The
application of these enzymes in synthetic key steps will drastically
simplify the chemical parts of the total synthesis and enable the
production of previously inaccessible derivatives. Methodically, the
project comprises organic synthesis as well as cloning, expression
and engineering of enzymes. Interested candidates should have a
solid background in Organic Chemistry and an interest in learning
methods from enzymology and basic molecular biology.
Required qualification: Master in Chemistry or Life Science
Project A3: Combined mutasynthetic / total synthetic approaches
to Noricumazol libraries for improved anti-HCV properties
Prof. Andreas Kirschning, Leibniz University Hannover, Institute of
Organic Chemistry and Center of Biomolecular Drug Research (BMWZ)
Web address:
http://www.akoci.uni-hannover.de/AK_Kirschning/index.htm
The Noricumazols are secondary metabolites, recently isolated from
myxobacteria, whose structures were established by total synthesis.
It was discovered that Noricumazol A exhibits strong ion channel
inhibitory activity and it very effectively inhibits growth of the
hepatitis C virus thus being a lead candidate for further biological
evaluation. In cooperation with the Müller group, who will establish
and utilize several blocked mutants, mutasynthetic and total
synthetic strategies will be established to create libraries of
Noricumazol A. Biological evaluation conducted at TWINCORE
(Pietschmann group) in Hannover will be directed towards ion
channels such as the viral P7 channel as well as on HCV associated
with host cells.
Required qualification: Master in Chemistry
Project A4: Silphinenes and Penifulvins - A Biogenetic
Realtionship?
Dr. Tanja Gaich, Leibniz University Hannover, Institute of Organic
Chemistry
Web address:
http://www.gaich-lab.uni-hannover.de
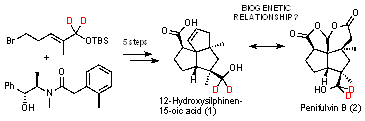
The project aims to synthesize deuterium labeled 12-hydroxy
silphinenic acid 1, feed it to its producer Penicillium griseovulvum
and investigate whether the deuterium containing 1 is transformed to
penifulvin B 2. Penifulvin B 2 as well as silphinenic acid 1, were
isolated from Penicillium griseofulvum indicating a biogenetic
relationship. A synthesis to the carbon skeleton of 2 was already
taken out in our laboratories following a biomimetic route.
Required qualification: Master in Chemistry
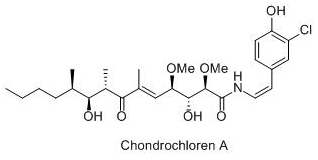
Project A5: Synthesis and biological evaluation of chondrochloren
Prof. Markus Kalesse, Leibniz Universität Hannover, Institut für
Organische Chemie
Web address:
http://www.kalesse.uni-hannover.de/
The aim of this project is to provide improved natural products that
interfere with bio-film formation. Our initial investigations
identified the secondary metabolite chondrochloren as promising
candidate to stop bio-film formation and to act as antibiotic
against gram-positive bacteria. This project will provide synthetic
access to this particular natural product and most importantly, to
derivatives and analogs to identify its structure-activity
relationship. This project collaborates with the project of Prof.
Irene Wagner-Döbler (Project C2), which will provide screens and
biological expertise to test these derivatives and analogs. Besides
generating analogs based on total synthesis, we will use the very
natural product, provided by fermentation as starting point to
generate derivatives. Additionally, this project aims at using a
combined synthetic-biosynthetic approach. By synthesis, the
corresponding acid could be provided and subsequently transformed to
generate different amide derivatives.
Required qualification: Master in Chemistry
Project A6: Photoactivatable Building Blocks for Microbial
Biosynthesis
Prof. Thomas Lindel, TU Braunschweig, Institut für Organische Chemie
Web address:
http://www.oc.tu-bs.de/lindel
Natural products are special, because their structures have evolved
under evolutionary pressure. However, the biological functions and
targets of most natural products still remain unknown, in particular
on the cellular or molecular level. The situation could be improved
if photoactivatable analogs of natural products would be available
in greater structural variety than presently. On irradiation inside
the cell, noncovalent natural product-target complexes would be
rendered covalent after bioorthogonal irradiation and, thus, become
analyzable by advanced mass spectrometry. A key problem concerns the
introduction of suitable photoactivatable functional groups, which
should be as small as possible in order to minimize structural
alteration of the candidate natural product. The research project
uniquely combines synthetic chemistry and microbial biotechnology
(cooperation with B4 - Dr. Dickschat) aiming at the incorporation of
diazirine moieties into structurally complex natural products. In
particular, it will be investigated to which extent terpene cyclases
are capable of converting diazirine-containing geraniol and farnesol
analogs to cyclic terpenes very difficult to access otherwise. For
non-ribosomal peptide synthesis, new photo amino acids will be
synthesized and investigated as possible substrates of the microbial
biosynthetic machinery.
Required qualification: Master in Chemistry
Project A7:
Unusual fatty acids as antimicrobial agents
Prof. Stefan Schulz, TU Braunschweig, Institut für Organische
Chemie
Web address:
http://www.oc.tu-bs.de/schulz/index_en.html
Unusual fatty acids have a high potential for biological activity
because they can interfere with common processes in living
organisms. Of special interest are compounds like
crepenynic acid with unusal
structural elements, in this case a triple bond. In the group
of Prof. Müller several new unusual fatty acids have been found with
interesting biological activity. In the project several of these
acids and their analogs will be synthesized as well as their
activity evaluated. The goal is to identify structural motifs
responsible for the observed effects. Furthermore, the biosynthesis
of these compounds will be analyzed together with the Müller group
and the necessary labeled precursors will be synthesized.
Required
qualification: Master in Chemistry
|