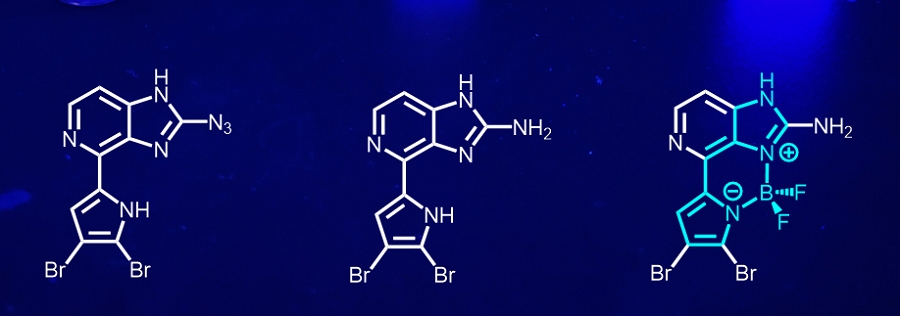
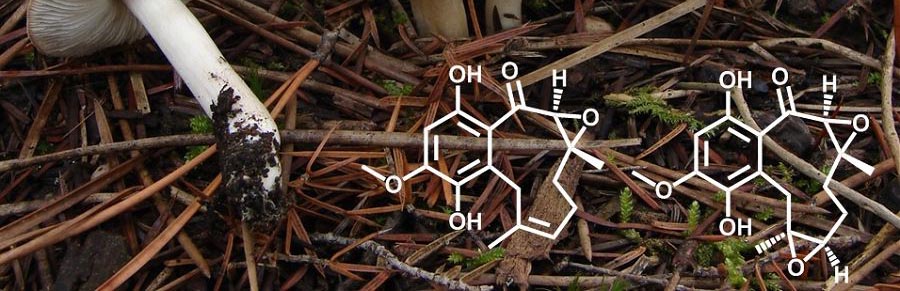
Why natural products?
Natural products are of prime importance for chemistry and for many neighboring disciplines such as biology and, further on, medicine. It is assumed that every natural product has been optimized by evolution towards a biological function. For instance, nicotine acts as an insecticidal and protects the young plant. The key metabolite oroidin does the same for marine sponges. Other natural products serve the chemical communication among organisms, such as insect pheromones. Increasingly, the protein binding partners of natural products are being discovered. This promises to become a new El Dorado of natural product research.
Why natural product chemistry?
Isolation and structure elucidation, partial and total synthesis, reactivity and functionalization stand at the heart of natural product chemistry. Among natural products, one finds a structural complexity that surpasses that of other molecules. Natural products may exhibit particular reactivities that are caused by unique architecture. This is fascinating for us as chemists and perhaps the true driving force behind natural product chemistry.
Why natural product synthesis?
The key goal of total synthesis is making a natural product available for interdisciplinary studies. As research progresses, total syntheses of interesting molecules become more and more elaborate, ideally (and never) leading to quantitative one-step processes starting from readily available starting materials. Thus, total synthesis frequently goes hand in hand with the development and testing of new synthetic steps that shorten existing sequences. The chemist is an architect. Total synthesis of natural products is also the main cause of structure revision, and the touchstone of synthetic methods.
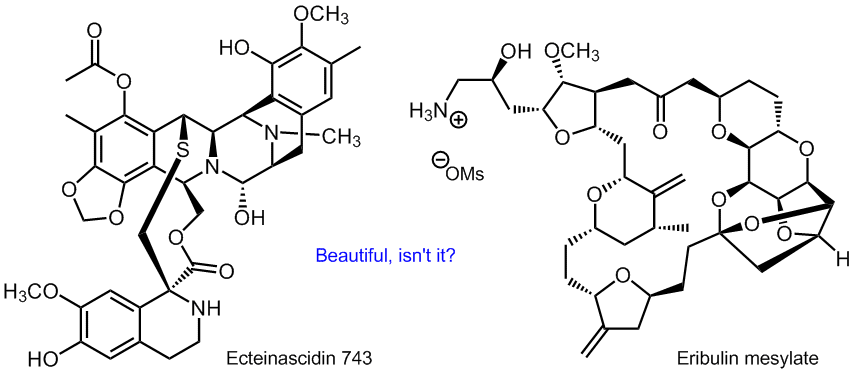
There are natural product-based drugs being produced by total synthesis on the market. An example is the anticancer agent eribulin mesylate, an analog of the marine natural product halichondrin B, that is made industrially in a 62-step process with 34 steps in the longest linear sequence. Natural product synthesis may also start from precursors obtained by fermentation, as in the case of ecteinascidin 743 that is made from biologically produced cyanosafracin B by adding 18 synthetic steps.