Interested in a Master thesis?
There is plenty of opportunity to start working on a Bachelor or Master thesis in the Lindel research group, or to participate as an advanced research student. Bachelor theses topics are usually closely associated with doctoral student's topics. Students are encouraged to contact their laboratory assistants. We will then work out a clearly defined topic together.
Generally, Organic Synthesis will be at the center of a master thesis project.
However, is there anyone out there who might be interested in the following?
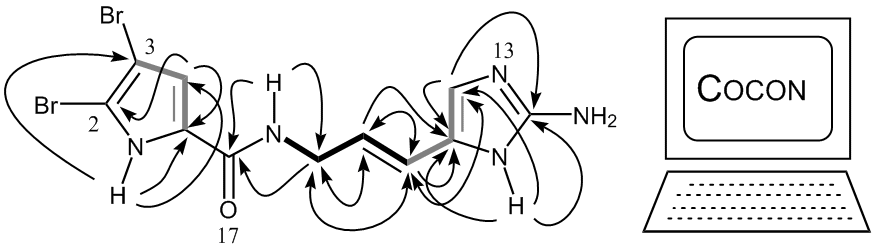
Software topic: HMBC-restricted structure generator
This is a computational project, but it is not about quantum chemical calculations. Goal is the programming of a computer software that would allow the generation of all structures being in agreement with a given molecular formula and 2D NMR-derived connectivities. Interpretation of HMBC correlations has to include 4J and 5J HC couplings. Ideally, a chemistry-based ranking of the generated proposals is to be implemented already during the generation of the search tree. The software would be of help for solving many structure elucidation problems, in particular, it only a few signals in the 1H NMR spectrum are available. Any nerd out there?