What are we doing?
The Lindel laboratory explores the total synthesis of natural products. Choosing a natural product as subject of research can be driven by pure structure or by biological activity. For instance, the family of pyrrole-imidazole alkaloids from marine sponges impresses by astonishing skeleton diversity generated by cyclization or dimerization of a single parent compound, oroidin. Biological activity drives our research on peptide-type natural products, among which the potently cytotoxic marine natural products malevamide D or hemiasterlin could be mentioned. Sometimes, we even aim at structures that have been described as being unstable, such as the cytotoxic salarin C.
Alkaloids from sea and land
The presence of nitrogen often poses a special challenge to the synthetic chemist. Free amino groups may compete with ligands of catalysts, make heterocycles electron-rich and prone to oxidation, or simply enhance the polarity so that separation by chromatography becomes tedious. However, this should not be a reason to avoid nitrogen-containing compounds.
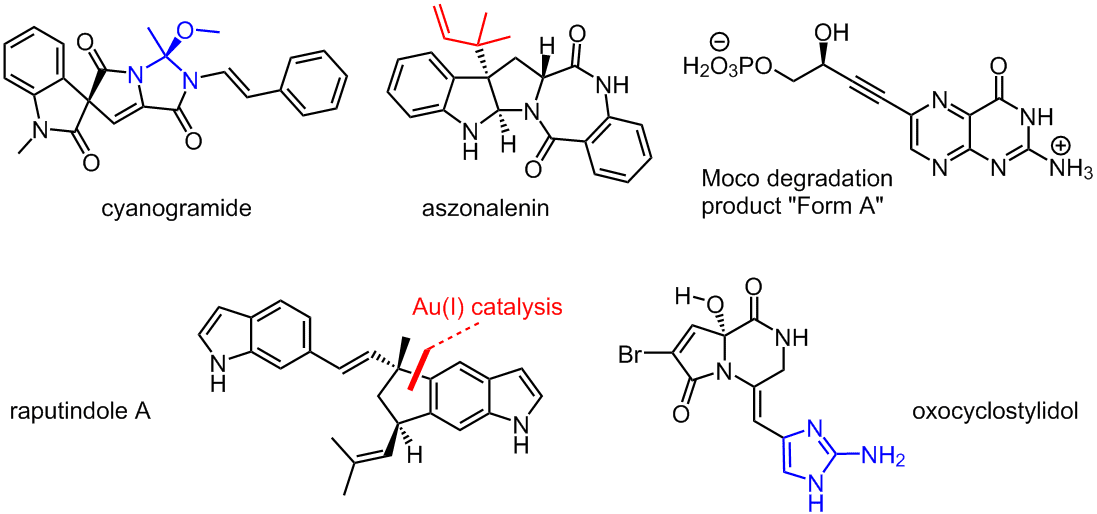
Current projects include the total synthesis of the indole alkaloids cyanogramide from the marine actinobacterium Actinoalloteichus cyanogriseus, aszonalenin from the fungus Aspergillus zonatus, and raputindole A from the tree Raputia simulans. The total synthesis of oxocyclostylidol from the marine sponge Stylissa caribica is challenging, because it contains an electron-rich 2-aminoimidazole unit in the presence of an oxidized pyrrole carboxamide partial structure. "Form A" is a degradation product of the molybdenum cofactor that has to be provided by synthesis as a standard for the quantification of Moco content in redox enzymes.
Peptides and functionalized amino acids
Peptides are often characterized by pronounced biological activity, in particular by cytotoxicity. Our program aims at providing large quantities by total synthesis for advanced biochemical studies. This includes the synthesis of rather complex amino acid-derived building blocks.
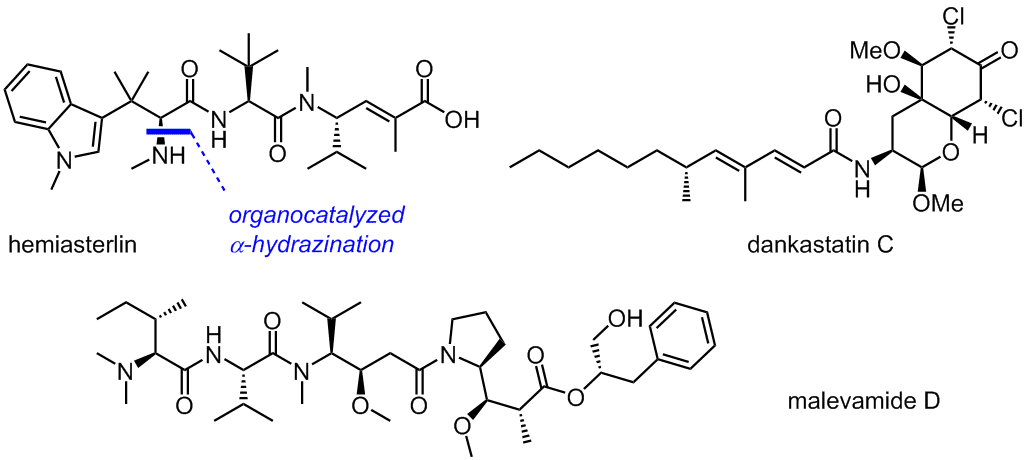
Hemiasterlin from the marine sponge Cymbastela sp. and malevamide D from the marine cyanobacterium Symploca hydnoides are cytotoxic in the single-digit nanomolar range and bind to tubulin. Their photoreactive analogs represent ideal cases for studying the effectiveness and chemoselectivity of new photolabeling reactions at the target. The cytototoxic dankastatin C from the sponge-derived fungus Gymnascella dankaliensis features a challenging, highly functionalized chlorotyrosine-derived head and a non-polar tail.
Polyketides and terpenoids
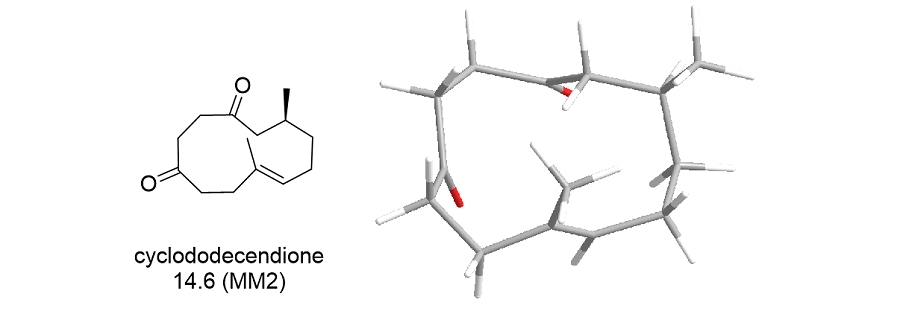
There is a also a number of very interesting terpenoids and polyketides that we are working on. We prefer medium-sized or larger rings that ought to be cyclized. Macrocyclic structures have shown a preference to bind at the interface of protein subunits.
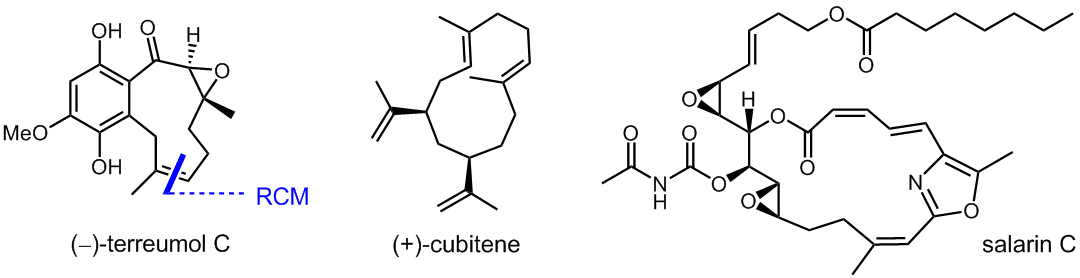
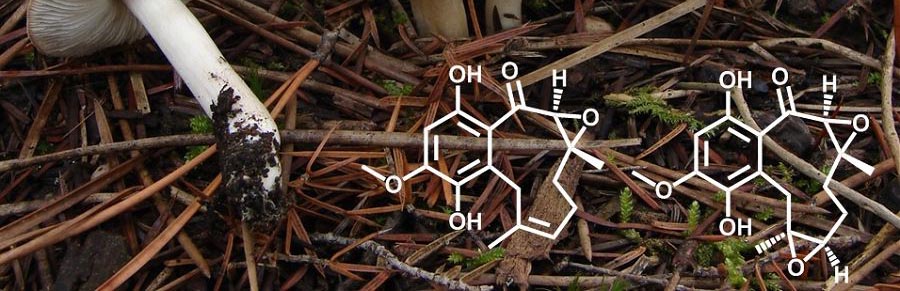
The terreumols from the edible fungus Tricholoma terreum are, in part, accessible by ring closing metathesis forming the ten-membered ring. Currently, we study their post-synthesis functionalization. Cubitane-type diterpenoids, as represented by cubitene from the termite Cubitermes umbratus, constitute a unique small group of diterpenoids that mainly occur in marine corals. On the polyketide side, we endeavour the total synthesis of several of the salarins from the sponge Fascaplysinopsis sp., among which salarin C is the most cytotoxic, and the most unstable.
Photoreactive heterocycles
As a relatively new branch of our research, we explore the chemistry of photoreactive heterocycles, aiming at their use as tool for the discovery of new biochemical targets of natural products. In the current phase of the project, it is the chemistry that puzzles us.
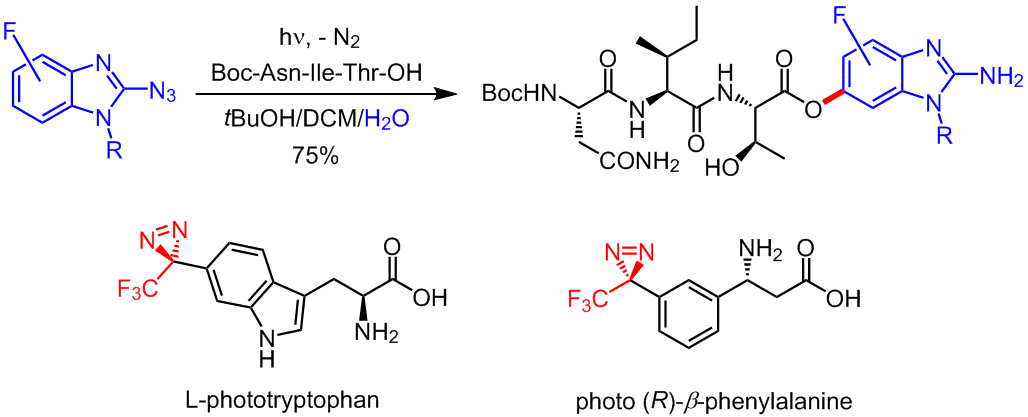
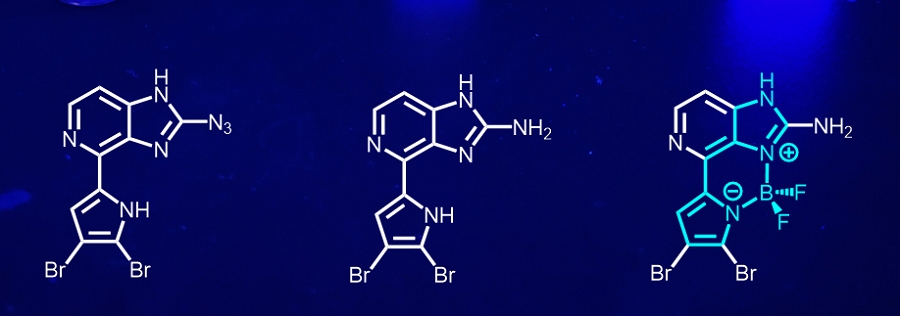
We synthesized and studied diazirine-based analogs of amino acids, such as L-phototryptophan and photo-beta-phenylalanine, ready to be incorporated into peptide analogs. Aiming at higher chemoselectivity, we discovered the photoarylation of carboxylic acids on irradiation of 2-azidobenzimidazoles. This adds a new player to the list of PAL reagents.